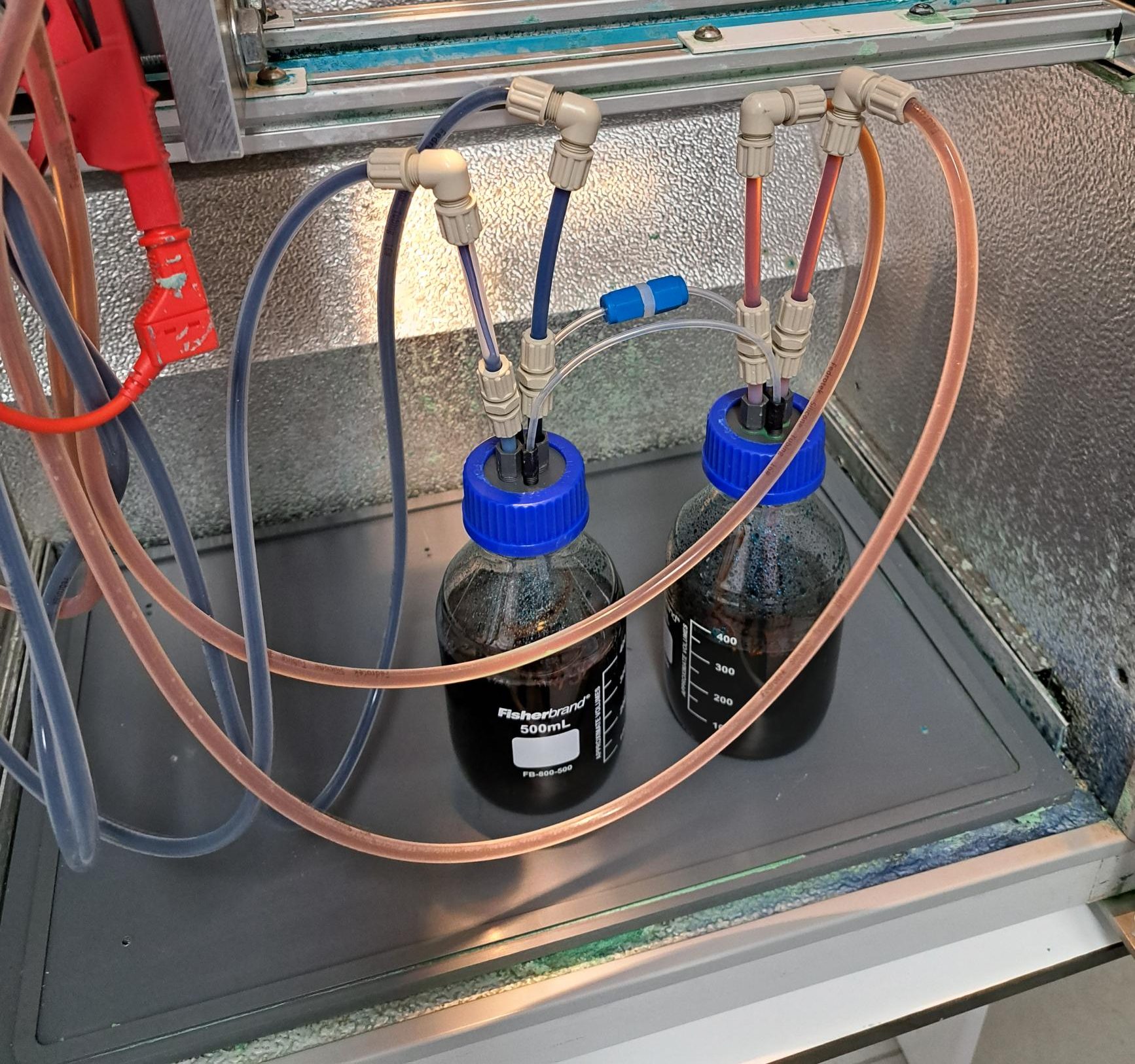
As part of the TÜV certification process—and being the core of the battery—the vanadium electrolyte must be thoroughly characterized. This includes the analysis of various physicochemical properties as well as its flammability. While this may seem like a routine task, the characterization must be performed not only on the fully discharged electrolyte, but also on the positively charged and discharged states.
To reach a high state of charge (SOC >95%), one of the tanks must be set as the capacity-limiting side, allowing it to reach a higher SOC compared to the other tank. Moreover, a constant current charging protocol must be followed by a constant voltage step to ensure maximum conversion of the active species (in this case, vanadium III and IV). Using this approach, we can generate and store highly concentrated solutions of vanadium V and vanadium II.
Vanadium V is considered the most aggressive species in the electrolyte. As a strong oxidizing agent—comparable to hydrogen peroxide (H₂O₂)—it can significantly contribute to the degradation of components such as frames, membranes, and bipolar plates (BPPs).
On the other hand, characterizing vanadium II is particularly challenging, as it is highly sensitive to oxygen. Both the solution and the surrounding environment must be deoxygenated before preparation and experimentation. This species represents the most reducing state of the vanadium electrolyte and is known to contribute to the deactivation of carbon felts.
This characterization of high-SOC solutions is not only essential for TÜV certification but also serves as a valuable tool for accelerated aging tests of various battery components, including membranes, carbon felts, and BPPs.
ACKNOWLEDGEMENT: This work was supported by the project: IPCEI_IE_FLOW_BESS_012021_2. phase